Recent advancements in microscopy have provided a breakthrough in cancer research, enabling scientists to closely observe and understand how antibodies identify and target cancer cells. This innovation, which could dramatically improve cancer treatment strategies, opens a new frontier in precision medicine. By revealing the intricate molecular interactions between antibodies and tumor cells, the technique promises to offer more effective and personalized therapeutic options for patients battling cancer.
The Role of Antibodies in Cancer Treatment
Antibodies are proteins produced by the immune system to recognize and neutralize foreign substances, such as bacteria and viruses. Over the years, researchers have harnessed this natural ability to develop monoclonal antibodies, a type of antibody engineered in laboratories to target specific proteins or antigens present on cancer cells. These antibodies can bind to cancer cells, flagging them for destruction by the immune system or directly inhibiting their growth.
One of the major challenges in cancer therapy, however, is understanding the precise mechanisms by which antibodies recognize and interact with cancer cells. While some therapies have shown promise, the variability in how patients respond has kept researchers seeking better ways to predict and improve treatment outcomes.
The Microscopy Breakthrough
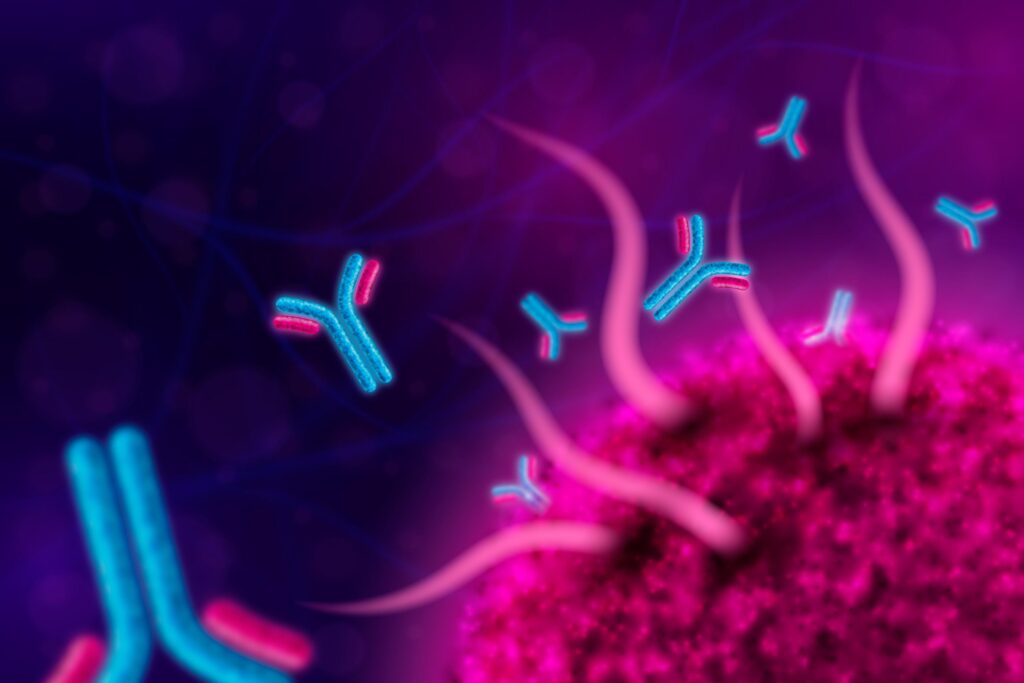
A newly developed microscopy technique is allowing researchers to observe, in unprecedented detail, how antibodies interact with cancer cells at the molecular level. This new method, known as super-resolution microscopy, offers a level of imaging that surpasses the capabilities of traditional microscopy. Unlike conventional methods that are limited by the diffraction of light, super-resolution microscopy enables scientists to visualize structures at the nanoscale, which is crucial for studying the fine interactions between antibodies and cancer cells.
By using this technique, researchers can directly observe how antibodies bind to specific proteins on the surface of cancer cells, a critical step in targeted therapy. They can track the movement and binding process in real-time, as well as monitor how these interactions evolve under different conditions or in response to treatment. The ability to observe such intricate details opens up a wealth of opportunities for refining antibody-based therapies.
Mechanisms of Antibody Action
The breakthrough microscopy technique has also provided valuable insights into the mechanisms by which antibodies target and destroy cancer cells. One of the key observations from this research is the identification of specific regions on cancer cell surfaces where antibodies tend to bind. These areas, known as epitopes, are unique to cancerous cells and differ from those on normal, healthy cells.
Antibodies typically function by binding to these cancer-specific epitopes, marking the cells for destruction by the immune system’s T-cells or macrophages. The new microscopy technology allows researchers to visualize how antibodies form complexes with these epitopes, triggering immune responses that lead to cell death.
In addition to helping researchers better understand the immune response, this technique can also shed light on why some antibodies are more effective than others. The precise positioning of antibodies on cancer cells, their interaction with surrounding molecules, and the formation of these immune complexes all contribute to the effectiveness of the therapy.
Moreover, scientists have been able to track how antibodies evolve over time when introduced to different types of cancer. For example, they observed how the surface proteins on cancer cells might change in response to antibody treatments, allowing them to adapt or “escape” the immune response. This phenomenon, known as immune evasion, can explain why some patients develop resistance to antibody-based therapies. Understanding these dynamics is key to developing more robust and adaptable treatments.
Implications for Cancer Treatment
The insights gained from this revolutionary microscopy technique could have profound implications for cancer treatment. With a clearer understanding of how antibodies work at the molecular level, researchers and clinicians can fine-tune existing therapies for better efficacy. This is particularly important in the context of personalized medicine, where treatments are tailored to the unique genetic makeup of both the patient and their cancer.
Additionally, the technique can help in the design of novel antibody-based drugs. By identifying the exact binding sites and mechanisms that lead to the most effective tumor targeting, scientists can engineer antibodies that are more potent, more selective, and less likely to cause off-target side effects. This could significantly reduce the toxicity and side effects often associated with traditional cancer treatments, such as chemotherapy.
Furthermore, the technique might also aid in monitoring cancer progression and treatment response. By observing how antibodies interact with cancer cells in real-time, clinicians can assess whether the treatment is effective or if the cancer is developing resistance. This allows for more dynamic and responsive treatment plans, improving patient outcomes.
Potential for Combination Therapies
Another exciting prospect of this breakthrough is the potential for combination therapies. Cancer is a complex disease, and using a single therapeutic approach may not always be sufficient. Combining antibody treatments with other modalities, such as checkpoint inhibitors or targeted small molecules, could lead to synergistic effects, where the combination is more effective than any individual therapy.
For instance, the new microscopy technique could allow researchers to observe how antibodies interact with other therapies on the cancer cell surface. By understanding these combined interactions at a deeper level, they can optimize treatment regimens and design therapies that work better together. This could lead to more comprehensive cancer treatments with higher rates of success.
Challenges and Future Directions
Despite the excitement surrounding this new microscopy technique, there are still challenges ahead. One of the main hurdles is the technical complexity of the method, which requires highly specialized equipment and expertise. Furthermore, while the ability to observe antibodies in action is groundbreaking, translating these findings into clinical practice will require further research and validation.
Additionally, there is still much to learn about the full range of interactions between antibodies and cancer cells. Cancer is not a single disease but a collection of conditions, each with its own set of mutations and characteristics. The microscopy technique will need to be adapted and refined to study different types of cancer and how they respond to various antibody therapies.
In the coming years, as the technology becomes more accessible and advanced, it is expected that this microscopy technique will play an integral role in advancing cancer research and treatment. With continuous innovation, we may one day see treatments that are more precisely tailored to individual patients, leading to better outcomes and, ultimately, a cure for many forms of cancer.
Conclusion
The breakthrough microscopy technique offers an unprecedented look at how antibodies target and interact with cancer cells, providing insights that could revolutionize the future of cancer therapy. By uncovering the intricate molecular mechanisms behind antibody-based treatments, researchers can refine and develop more effective therapies. This discovery marks an exciting step forward in the quest for personalized and precision cancer treatment, holding great promise for improving the lives of patients worldwide.