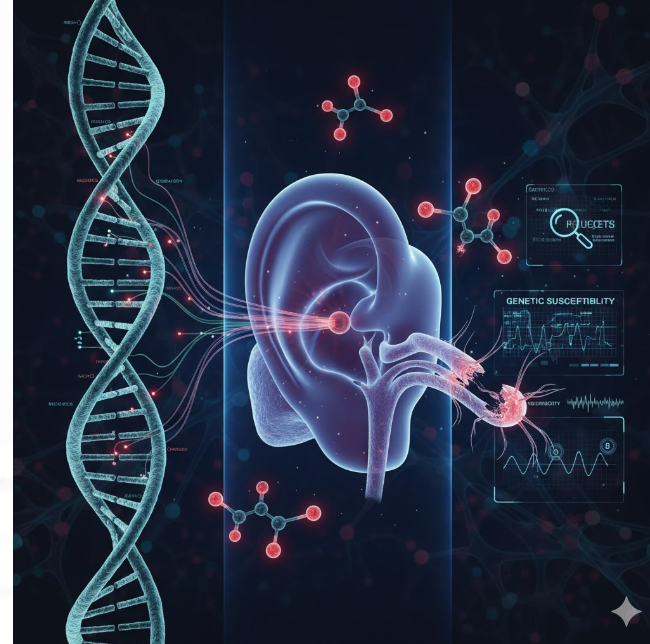
The vestibular system — our body’s internal compass for balance and spatial orientation — is one of the most delicate sensory systems in the human body. Damage to this system can result in vertigo, dizziness, and chronic imbalance, profoundly affecting quality of life. One of the leading causes of vestibular dysfunction is exposure to ototoxic drugs, a class of medications that harm the inner ear’s sensory structures. Common examples include aminoglycoside antibiotics, certain chemotherapeutic agents like cisplatin, and loop diuretics.
However, a puzzling observation has emerged: not everyone exposed to these drugs develops vestibular toxicity. While dosage and duration play a role, recent research reveals that genetic variations are a critical determinant of individual susceptibility. Mutations in genes related to mitochondrial function, drug metabolism, and cellular defense mechanisms can amplify or mitigate the toxic effects of these compounds. Understanding these genetic factors offers a path toward personalized medicine — one where clinicians can predict risk, tailor drug selection, and design protective interventions.
This article delves into the genetic architecture underlying vestibular system damage from ototoxic drugs, exploring the molecular pathways, biomarkers, and future therapeutic implications that shape this emerging field.
Understanding Ototoxicity: When Life-Saving Drugs Turn Harmful
Ototoxicity refers to the toxic effect of certain drugs on the inner ear’s cochlear and vestibular systems. While often unavoidable in critical care, these drugs can cause irreversible hearing loss or balance dysfunction. The vestibular system — comprising semicircular canals, otolith organs, and neural pathways — is especially vulnerable because of its high metabolic demand and sensitivity to oxidative stress.
Aminoglycosides, such as gentamicin and streptomycin, are among the most notorious ototoxic agents. They generate reactive oxygen species (ROS) that damage sensory hair cells in the vestibular epithelium. Similarly, chemotherapy agents like cisplatin induce mitochondrial DNA (mtDNA) damage and disrupt ionic homeostasis in the inner ear. Yet, clinical outcomes vary dramatically: two patients receiving identical doses may experience completely different side effects.
This discrepancy points toward a genetic predisposition to ototoxic damage. Specific allelic variants in genes responsible for antioxidant defense, mitochondrial stability, and drug transport may render some individuals more vulnerable. The study of these genetic underpinnings — collectively known as pharmacogenomics — offers unprecedented insight into why certain patients develop vestibular disorders while others remain unaffected, paving the way for precision prevention and intervention.
The Mitochondrial Genome: The Silent Determinant of Vestibular Susceptibility
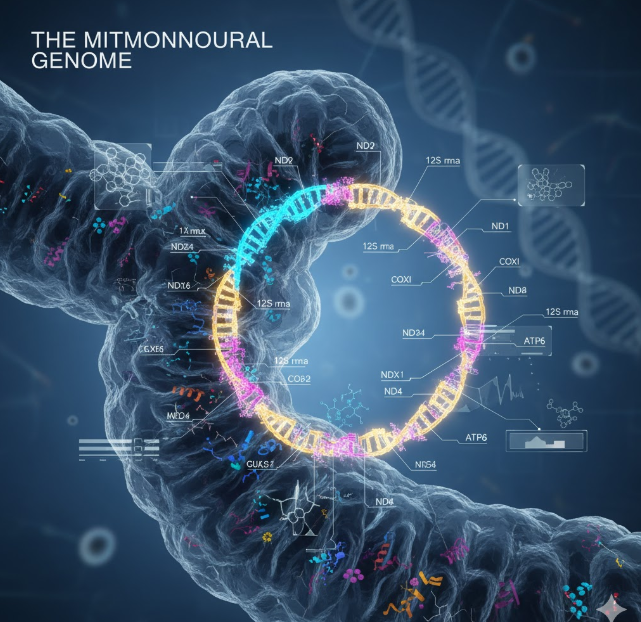
Among the most extensively studied genetic factors in ototoxicity are mitochondrial DNA mutations. The vestibular hair cells rely heavily on mitochondrial energy metabolism to maintain ionic gradients and signal transmission. Any disruption in mitochondrial function can therefore make these cells more prone to oxidative injury.
One of the best-known mutations linked to ototoxic susceptibility is m.1555A>G in the 12S rRNA gene. This variant alters the mitochondrial ribosomal RNA, causing aminoglycosides to bind more readily to mitochondrial ribosomes. As a result, even therapeutic doses can trigger excessive ROS production and apoptotic cell death in hair cells. Individuals carrying this mutation are at exceptionally high risk of both auditory and vestibular toxicity, even with a single exposure.
Other mitochondrial variants, such as m.1494C>T and m.7445A>G, have also been implicated in increased sensitivity to aminoglycosides. Because mitochondrial DNA is maternally inherited, these mutations often cluster within families, making genetic screening a valuable preventive measure.
Recent research has also shown that mitochondrial haplogroups — groups of related mtDNA lineages — can influence susceptibility, suggesting population-specific risk profiles. Understanding these mitochondrial influences not only clarifies mechanisms of vestibular damage but also supports the case for genetic screening before initiating ototoxic therapy, particularly in high-risk populations.
Nuclear Gene Variations and Their Role in Ototoxic Vestibulopathy
While mitochondrial mutations have received substantial attention, nuclear genes also play a critical role in determining the extent of vestibular system damage. Genes that regulate oxidative stress response, drug detoxification, and cellular repair pathways are particularly influential.
Variants in glutathione S-transferase (GST) genes, such as GSTM1 and GSTT1, affect the cell’s ability to neutralize reactive oxygen species. Individuals with deletions in these genes may have reduced antioxidant defenses, allowing ototoxic drugs to inflict greater mitochondrial and membrane damage in vestibular cells. Similarly, polymorphisms in superoxide dismutase (SOD) and catalase (CAT) genes can weaken the cellular response to oxidative stress, enhancing susceptibility to drug-induced injury.
Drug transporters also play a genetic role. The megalin (LRP2) gene, expressed in the inner ear’s marginal cells, mediates drug uptake. Variants increasing megalin expression may enhance aminoglycoside accumulation, elevating toxicity risk. Meanwhile, genes like TPMT (thiopurine methyltransferase) and ABCC1 influence detoxification capacity and cellular efflux, respectively, affecting how long these compounds remain active in sensitive tissues.
Together, these nuclear genes form a protective—or sometimes permissive—network that determines the resilience of the vestibular apparatus. Genetic mapping of these variants can thus guide clinicians in identifying vulnerable patients and optimizing drug regimens to prevent permanent vestibular dysfunction.
Genetic Modulation of Inflammatory and Apoptotic Pathways
Beyond oxidative stress, inflammation and apoptosis play a key role in vestibular system degeneration. Genetic differences in these pathways can significantly alter individual outcomes following exposure to ototoxic agents.
For instance, polymorphisms in TNF-α and IL-1β genes have been associated with exaggerated inflammatory responses within the inner ear. Elevated cytokine expression triggers microvascular changes, compromises the blood-labyrinth barrier, and accelerates the death of sensory hair cells. Genes regulating apoptotic proteins, such as BCL2, CASP3, and TP53, further determine whether a cell survives or succumbs under toxic stress.
In some individuals, hyperactivation of the p53 pathway has been observed following cisplatin exposure, promoting premature cell death in vestibular epithelia. Conversely, certain protective alleles upregulate BCL2, enhancing cell survival. These findings demonstrate how subtle genetic differences in cellular stress response can drastically change the outcome of drug exposure.
Epigenetic regulation adds another layer of complexity. DNA methylation and microRNA expression patterns influence gene transcription related to oxidative defense and apoptosis. This means that even in the absence of direct mutations, environmental and epigenetic factors can “switch on” or “off” genes that influence vestibular vulnerability. Understanding these molecular switches is crucial for developing drugs that can precondition or protect vestibular tissues against ototoxic injury.
Genetic Screening and Predictive Biomarkers for Ototoxic Risk
As the genetic basis of vestibular ototoxicity becomes clearer, the focus is shifting toward predictive genetic testing and biomarker discovery. Pre-treatment screening can identify individuals with high-risk genotypes, enabling physicians to modify therapy or adjust dosages accordingly.
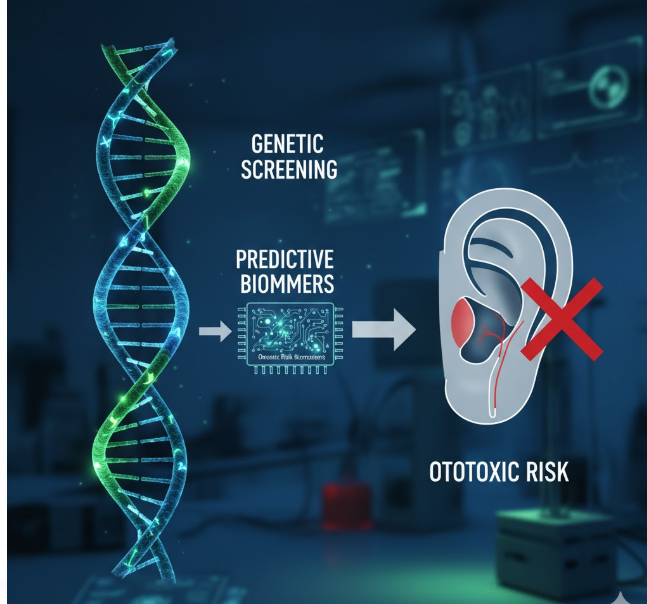
Screening for the m.1555A>G mutation is already recommended in some clinical settings before administering aminoglycosides. Similarly, multiplex genetic panels now include nuclear variants in GST, SOD, and CAT genes to predict oxidative vulnerability. In oncology, pharmacogenomic testing for TPMT and ABCC1 variants can guide cisplatin dosing to minimize ototoxic outcomes.
Beyond genetic sequencing, researchers are exploring molecular biomarkers — such as circulating mitochondrial DNA fragments or oxidative stress markers — that can indicate early vestibular injury even before clinical symptoms appear. The combination of genomic and proteomic data could enable real-time monitoring of patients at risk, allowing timely intervention.
In the near future, artificial intelligence models may integrate these multi-omic datasets to generate personalized ototoxicity risk scores. Such predictive systems could revolutionize patient safety by allowing precision dosing, alternative drug selection, and proactive vestibular protection in genetically susceptible populations.
Toward Genetic Protection and Future Therapeutic Directions
The ultimate goal of understanding genetic susceptibility is not merely prediction, but protection. As we map the genetic determinants of vestibular ototoxicity, new therapeutic strategies are emerging to counteract these effects at the molecular level.
Gene therapy approaches are being explored to enhance mitochondrial resilience by repairing or bypassing defective genes such as 12S rRNA. Antioxidant gene delivery systems, using vectors to upregulate SOD or CAT expression locally within the inner ear, show promise in experimental models. Another exciting avenue involves pharmacological preconditioning — using mild oxidative stress or natural antioxidants to activate protective genetic pathways before administering ototoxic drugs.
CRISPR-based technologies may soon allow correction of high-risk mutations in stem cell-derived vestibular tissues, potentially restoring balance function in affected individuals. Furthermore, combining genetic insights with regenerative medicine could lead to the development of personalized vestibular implants or targeted therapies that rebuild damaged sensory epithelium.
For now, integrating genetic counseling into ototoxic drug protocols remains a crucial step. Educating clinicians about genetic variability can reduce the incidence of preventable vestibular damage and empower patients to make informed choices. As genetic research deepens, the vision of safe, individualized therapy — where drugs heal without harming — becomes increasingly attainable.
Conclusion
The intersection of genetics and ototoxicity research has transformed our understanding of vestibular system vulnerability. No longer seen as random side effects, vestibular complications are increasingly recognized as genetically influenced phenomena. By identifying key mutations, mapping cellular pathways, and integrating genetic screening into clinical practice, we move closer to personalized, preventive medicine. The future lies not in avoiding essential drugs, but in using genetic insights to protect the body’s delicate sense of balance — ensuring that lifesaving treatments no longer come at the cost of equilibrium.
Also read-