Pregnancy loss is a devastating, common occurrence, affecting up to a quarter of all pregnancies. While most losses are isolated events, when a woman experiences three or more consecutive clinical pregnancy losses before 20 weeks gestation, it is medically classified as Recurrent Pregnancy Loss (RPL). RPL is a complex, heartbreaking condition that impacts 1% to 3% of couples trying to conceive, subjecting them to a cycle of hope, loss, and profound emotional distress.
Approximately 50% of all early pregnancy losses are attributed to genetic or chromosomal abnormalities in the fetus. However, even after extensive testing of the parents, the cause of RPL often remains unknown—a frustrating diagnosis that leaves couples without answers and clinicians without a clear path for intervention. This large group of “unexplained RPL” represents a critical gap in reproductive medicine.
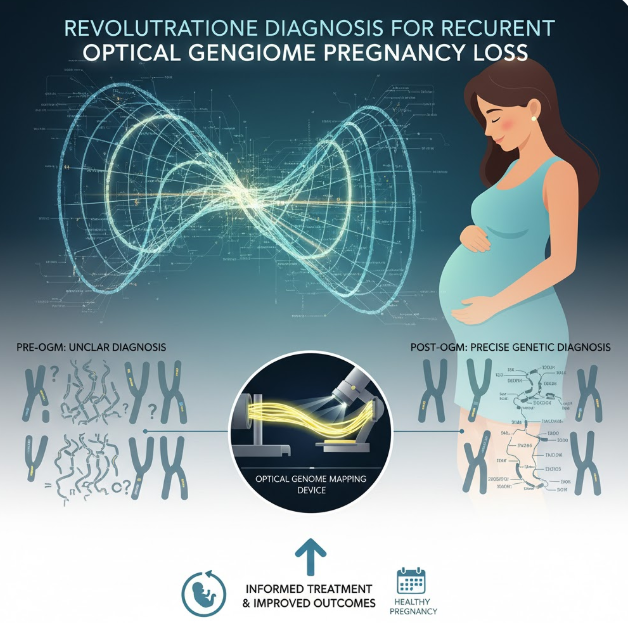
In the quest to close this diagnostic gap, molecular pathology is constantly evolving. A cutting-edge technique known as Optical Genome Mapping (OGM) is now emerging as a revolutionary tool. OGM provides an unprecedented, high-resolution view of the entire human genome’s structure, allowing researchers and clinicians to finally identify subtle yet significant genetic abnormalities that have long been the hidden drivers of RPL. Recent studies presented at major scientific conferences, such as the Association for Molecular Pathology (AMP) 2025 Annual Meeting & Expo, confirm that OGM is poised to transform the diagnostic landscape for couples struggling with this complex disorder.
The Diagnostic Dilemma: Limitations of Traditional Testing
For decades, the standard approach for evaluating potential genetic causes of RPL in parents has relied on two primary methods: conventional karyotyping and chromosomal microarray analysis (CMA). While essential, both methods have significant limitations that contribute to the high rate of unexplained RPL.
Karyotyping involves analyzing the number and structure of chromosomes under a microscope. It is effective at detecting large chromosomal abnormalities, such as aneuploidies (missing or extra chromosomes) or major balanced translocations. However, its resolution is low. It can easily miss small, sub-microscopic structural variations (SVs)—deletions, duplications, or complex rearrangements—that are smaller than 5-10 megabases. More critically, karyotyping relies on cells that can be successfully cultured and requires significant expertise in visual analysis, which can be prone to subjective interpretation.

Chromosomal Microarray Analysis (CMA) offered an increase in resolution, making it the preferred method for detecting copy number variations (CNVs)—deletions or duplications of genetic material—across the genome. CMA is highly effective at identifying the gain or loss of DNA segments. However, a major weakness of CMA in the context of RPL is its inability to detect balanced structural rearrangements. Balanced rearrangements occur when chromosomes break and swap segments, or when an entire segment is inverted, but no net genetic material is gained or lost. Since the patient carrying a balanced rearrangement is usually clinically healthy, this abnormality is often silent and inherited. Yet, when passed to offspring, it leads to an unbalanced genome, causing recurrent miscarriage. CMA simply cannot ‘see’ these crucial, balanced inversions or translocations.
It is this gap—the inability of standard tests to reliably detect small-scale structural variations and balanced rearrangements—that leaves so many couples trapped in the frustrating category of unexplained RPL. The structural integrity of the genome, often overlooked by sequencing-based methods, is paramount to successful reproduction, making a high-resolution, structure-focused technology imperative.
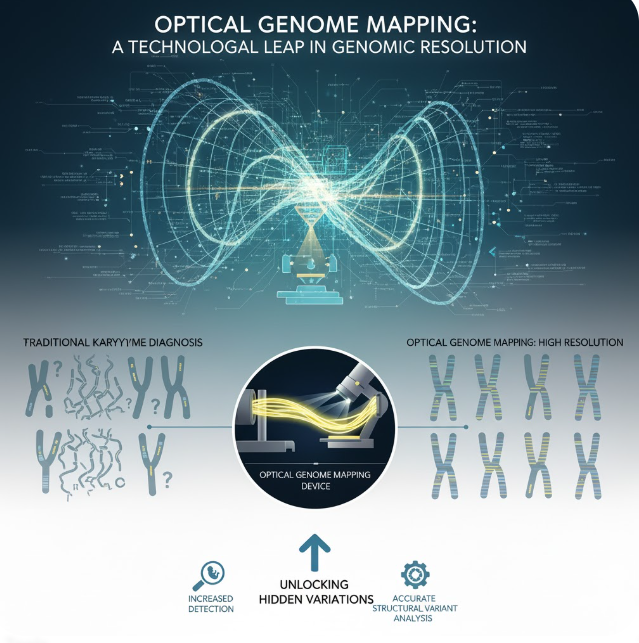
Optical Genome Mapping: A Technological Leap in Genomic Resolution
Optical Genome Mapping (OGM) represents a paradigm shift in how structural changes in the genome are detected and analyzed. Instead of fragmenting DNA and reading short sequences (like in traditional sequencing), OGM focuses on analyzing long, intact DNA molecules.
The process involves extracting ultra-high molecular weight (UHMW) DNA from a patient’s cells. This DNA is then labeled with fluorescent tags at specific sequence motifs along its length. These long, tagged molecules are straightened and passed through nanofluidic channels on a chip, allowing specialized cameras to image and record the precise fluorescent pattern of each molecule. By comparing the resulting “barcode” pattern of the patient’s genome to a known reference genome, researchers can rapidly and accurately identify virtually all types of Structural Variations (SVs), ranging from 500 base pairs to entire chromosome arms. This includes:
- Large deletions and duplications (CNVs).
- Inversions (sections of DNA flipped).
- Translocations (segments swapped between non-homologous chromosomes).
- Complex rearrangements that involve multiple break points.
Crucially, because OGM directly visualizes the long-range order of the DNA molecule, it is highly adept at detecting the balanced structural rearrangements that CMA misses and often requires the subjective expertise of karyotyping. OGM delivers a comprehensive, objective, and high-throughput map of the genome’s structural landscape, providing a resolution that far surpasses conventional methods.
Study 1: Revealing Hidden Structural Variants in RPL Patients
The true power of OGM in the context of reproductive health was highlighted by the work of researchers at Dartmouth-Hitchcock Medical Center. Their investigation centered on a direct comparison: could OGM detect harmful chromosomal changes in RPL patients who had already undergone standard genetic testing (karyotyping or CMA) that yielded non-diagnostic results?
The study provided compelling evidence that OGM significantly enhances the diagnostic yield. By carefully reviewing the OGM data from the patient cohort, researchers discovered an average of approximately 40 structural changes in the genome of each individual. While many of these changes were benign or previously unknown variants of uncertain significance, a significant number provided clear diagnostic links to the patient’s RPL history.
The study specifically focused on a panel of 238 genes already known to be associated with RPL and infertility. In multiple crucial cases, OGM detected structural changes—such as micro-deletions, inversions, or translocations—that directly disrupted or entirely affected these important RPL-related genes. These were genetic abnormalities that the previous, standard tests had completely failed to identify. For instance, the OGM analysis revealed a hidden chromosome rearrangement in one patient that disrupted critical genes, while in two other cases, four key RPL-related genes were directly impacted by novel structural changes.
The clinical implication is profound. The ability of OGM to reveal a clearer, more complete map of the patient’s structural genome dramatically changes the diagnosis from “unexplained” to “genetically linked.” For couples, this move from an ambiguous prognosis to a tangible, explained cause is transformative, allowing for more precise reproductive counseling, risk stratification, and potentially targeted preimplantation genetic testing (PGT) during assisted reproductive cycles. The authors concluded that using OGM alongside standard genetic tests is essential to comprehensively enhance the diagnostic evaluation of recurrent pregnancy loss.
Study 2: The Unappreciated Role of Chromosome Fragile Sites
Beyond the detection of standard structural variations, OGM is proving instrumental in characterizing some of the most complex and unstable regions of the genome: fragile sites. These are specific areas of human chromosomes that are inherently prone to developing breaks, gaps, or constrictions, particularly when the DNA is under replication stress. While their role in genomic instability is well-documented, their specific contribution to recurrent pregnancy loss has been historically underappreciated and difficult to study.
A separate investigation conducted by researchers at Queens University’s Kingston Health Sciences Centre and the University of Ottawa focused specifically on this connection. They examined the case of a 33-year-old patient who had suffered three consecutive early pregnancy losses. Initial traditional chromosome testing on this patient revealed breaks at a specific, rare fragile site known as FRA16B in roughly one-third of her cells. This finding suggested an intrinsic instability, but the underlying molecular cause remained elusive.
This is where OGM provided the definitive answer. Using the high-resolution mapping capabilities of OGM, the researchers were able to precisely map the structure of the FRA16B site. They discovered that the repeated DNA segment at FRA16B was unusually large in this patient. This expansion of the repetitive sequence provided the molecular confirmation of the instability, establishing a clear link between this genomic defect and her history of pregnancy loss.
This case study is vital because it demonstrates OGM’s ability to move beyond mere detection of a break to provide the molecular etiology—the specific structural anomaly—responsible for the instability. Fragile sites like FRA16B can easily be overlooked or poorly characterized by lower-resolution methods. By combining traditional cytogenetic testing (like karyotyping) with the precision of OGM, clinicians can gain a clearer and more precise understanding of these unstable genetic regions. The work strongly suggests that fragile sites are indeed a hidden and often-missed contributor to reproductive failure, a category that OGM is uniquely suited to identify.
Future Implications and Conclusion
The collective evidence from these and other emerging studies positions Optical Genome Mapping as a critical new tool in the reproductive genomics arsenal. It effectively addresses the major shortcomings of traditional diagnostics by offering a comprehensive, high-resolution view of the structural variants—both common and rare, balanced and unbalanced—that drive RPL.
For patients, the implications are vast. A molecular diagnosis offers closure and validates the physical and emotional toll of recurrent loss. More importantly, an accurate genetic diagnosis allows clinicians to move beyond general management strategies to provide personalized reproductive counseling.
Knowing the specific structural rearrangement allows genetic counselors to accurately calculate recurrence risk and discuss specific family planning options, such as using donor gametes, or utilizing Preimplantation Genetic Testing for Structural Rearrangements (PGT-SR) during in vitro fertilization (IVF) cycles. While the technology is still on its path toward widespread clinical adoption, the future trajectory is clear. As OGM becomes more accessible and integrated into standard clinical workflows, the percentage of RPL cases deemed “unexplained” will shrink significantly. The ability of OGM to accurately map the structural anomalies of the genome, from large translocations to subtle expansions at fragile sites, is not just a scientific victory—it is a promise of hope for the thousands of couples seeking answers and a successful pregnancy outcome. Optical Genome Mapping is truly mapping the way toward a more precise, evidence-based era in recurrent pregnancy loss diagnosis.