Alcohol Use Disorder (AUD), a chronic and relapsing brain disease, affects millions globally and represents a severe public health crisis. Despite decades of research, current pharmacological treatments for AUD are often met with limited success, high relapse rates, and debilitating side effects. The core challenge in treating AUD lies in its complex interplay with emotional states: heavy alcohol consumption over time alters brain circuits, particularly those responsible for processing stress, fear, and anxiety, creating a powerful feedback loop that drives compulsive drinking to alleviate negative emotional states.
However, a promising new therapeutic avenue is emerging from an unexpected source: psychedelics. Compounds traditionally known for their mind-altering properties are being intensely studied for their potential to “reset” deeply ingrained pathological brain patterns associated with various psychiatric conditions, including addiction. Specifically, a psychedelic found in certain mushrooms, and the compound the body converts it into, psilocin, is gaining attention as a potential treatment for AUD.
A groundbreaking preclinical study, led by Sarah Magee and Melissa Herman at the University of North Carolina at Chapel Hill (UNC) and published in The Journal of Neuroscience, sought to bridge the gap between this clinical promise and the underlying neurobiological mechanisms. Their work successfully identified a key population of neurons in the brain’s emotional center—the central amygdala (CeA)—that is targeted by psilocin, providing critical mechanistic insight into how this psychedelic compound might fundamentally alter the destructive cycle of alcohol addiction.
The Brain’s Emotional Hub: The Central Amygdala (CeA)
To understand the study’s findings, one must first appreciate the role of the central amygdala (CeA). The amygdala is a small, almond-shaped region deep within the temporal lobe, critically involved in processing memory, decision-making, and emotional reactions. The CeA, in particular, is a fundamental component of the brain’s “extended amygdala,” playing a disproportionately large role in stress response and negative emotional processing.
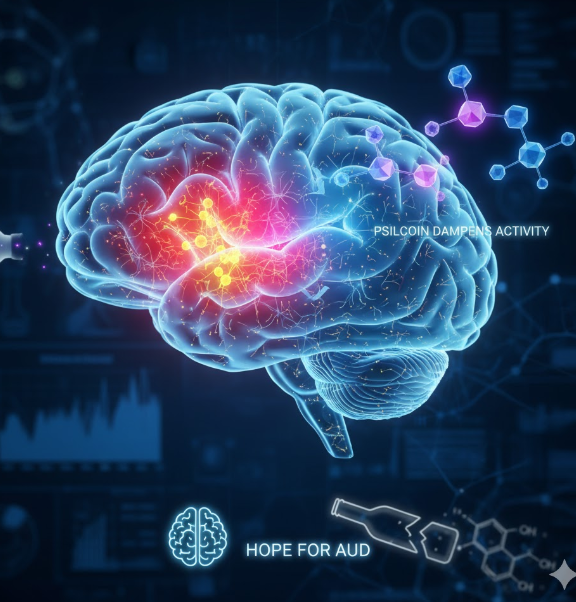
In the context of addiction, the CeA is a locus of pathological change. Chronic exposure to alcohol is known to increase the excitability and activity of specific neurons within the CeA. This heightened state of activity is strongly associated with the negative emotional symptoms—like anxiety, irritability, and stress—experienced during alcohol withdrawal and abstinence. The intense desire to extinguish these negative feelings is a powerful driver of relapse and compulsive seeking in AUD.
The UNC researchers focused their investigation on a specific population of neurons within the CeA that express the corticotropin-releasing factor receptor 1 (CRF-R1). CRF is a primary stress hormone, and CRF-R1-expressing neurons are integral to the body’s stress response system. Therefore, an overactive CRF-R1 neuronal population in the CeA is considered a neurobiological signature of anxiety, stress, and, crucially, alcohol-seeking behavior in individuals with AUD.
The Psilocin Intervention: Dampening the Stress Signal
The core hypothesis of the UNC team was that psilocin—the active, non-metabolized form of the psychoactive compound psilocybin—must exert its therapeutic effects by modulating the activity of these pathologically altered stress-related neurons.
Using female mice, which were intentionally selected for the study because they exhibit a greater propensity for alcohol consumption compared to male mice, the researchers established a model of long-term alcohol exposure. This exposure reliably leads to the characteristic high activity in the CeA neurons that mirrors the pathology seen in human AUD.
When the researchers introduced psilocin, the results were striking and provided direct neurobiological evidence:
- Neuronal Dampening: Psilocin was found to significantly dampen, or suppress, the activity of the CRF-R1 neurons in the central amygdala that had become hyperactive due to long-term alcohol exposure. This is the crucial mechanistic link—the drug reverses the pathological hyperactivity associated with chronic drinking.
- Behavioral Correlation: This decrease in neuronal activity was directly associated with a measurable reduction in alcohol drinking during the period of drug exposure. By effectively muting the stress-driven signal from the CeA, the mice’s motivation to consume alcohol to alleviate that stress was reduced.
As explained by Melissa Herman, one of the study’s lead researchers, “It makes sense that dampening this neuron population reduces drinking because increased activity in these neurons is associated with alcohol use disorders.” The study thus demonstrated that psilocin directly targets and calms the neuronal population responsible for driving the stress and anxiety component of addiction.
Molecular Pathways: The Role of Glutamate and GABA
While the summary provided by the source material focuses primarily on the resulting dampening of neuronal activity, a complete understanding of this mechanism in the CeA requires exploring the underlying neurochemical communication.
The activity of neurons in the CeA is finely balanced by two primary neurotransmitter systems:
- Glutamate: The brain’s main excitatory neurotransmitter, responsible for increasing neuronal activity.
- GABA (Gamma-Aminobutyric Acid): The brain’s main inhibitory neurotransmitter, responsible for decreasing or “dampening” neuronal activity.
Chronic alcohol exposure tips this balance. In AUD, the glutamatergic signaling in the extended amygdala is often dysregulated, and the overall balance shifts toward excessive excitation.
Psilocin, like other classic psychedelics, is a powerful agonist of the 5-HT2A serotonin receptor. While the 5-HT2A receptors are often associated with the hallucinogenic effects in the cortex, their action in deeper brain structures like the CeA can modulate the glutamate/GABA balance.
In the context of the CeA’s CRF-R1 neurons, the observed “dampening” by psilocin likely results from a combination of effects: perhaps by increasing inhibitory GABAergic input onto these hyperactive neurons, or by decreasing excitatory glutamatergic input to them. By re-establishing a healthier inhibitory tone within the CeA, psilocin can essentially normalize the emotional baseline of the animal, reducing the stress-induced drive to self-medicate with alcohol.
This suggests that the therapeutic utility of psilocin in AUD stems from its ability to acutely normalize the function of a key node in the brain’s emotional circuitry that has been pathologically altered by long-term alcohol exposure.
The Clinical Significance: Bridging Addiction and Emotional Health
One of the most compelling aspects of the UNC study is the overlap between AUD, anxiety, and depression. It is well-established that AUD frequently co-occurs with other psychiatric disorders, particularly major depressive disorder and various anxiety disorders. The CeA, with its critical role in emotional processing and stress, is a key hub involved in all three conditions.
The researchers highlighted that increased activity in the CeA’s CRF-R1 neurons is associated not only with alcohol use disorders but also with symptoms of depression and anxiety. Psilocin’s ability to dampen this specific neuronal population, even in mice with less severe alcohol exposure, strongly supports the clinical observations showing that psychedelics may help improve issues with emotional processing and stress across a range of psychiatric disorders.

This mechanistic insight offers a unified explanation for the broad therapeutic promise of psilocin: it may not be a treatment for AUD or anxiety, but a treatment for the shared underlying neurobiology of emotional dysregulation that drives both. By calming the brain’s stress center, the compound simultaneously addresses the emotional symptoms that drive drinking and the negative emotional states that constitute anxiety and depression. This is particularly significant for human trials, as a single treatment with a profound neurobiological “reset” could potentially address a cluster of co-occurring symptoms, simplifying treatment and improving patient outcomes.
Research Design Considerations: Focus on Female Mice
The deliberate focus on female mice in this preclinical study is another important and commendable aspect. Historically, neuroscience research, particularly in addiction, has often relied predominantly on male animal models. However, the neurobiological and behavioral responses to alcohol can differ significantly between sexes.
The researchers selected female mice specifically because they tend to drink more alcohol than their male counterparts in lab settings, providing a robust model for studying excessive consumption. This choice is vital for addressing the nuances of AUD, especially since women often progress more rapidly from initial drinking to dependency—a phenomenon sometimes referred to as “telescoping”—and may experience higher rates of co-occurring anxiety and stress. By focusing on female models, the study ensures that the mechanistic insights derived are relevant to a comprehensive understanding of AUD across all affected populations.
Future Implications for AUD Treatment
The work of Magee and Herman serves as a critical piece of the puzzle, emphasizing that preclinical research is necessary for filling the knowledge gaps regarding drug mechanisms, especially in the burgeoning field of psychedelic research.

The findings have profound implications for shaping clinical trial interpretations and guiding future drug development:
- Optimizing Dosage and Timing: Understanding the precise cellular target (CRF-R1 neurons in the CeA) allows researchers to better fine-tune psilocin dosage and timing protocols in human trials to maximize therapeutic effect while minimizing off-target effects.
- Developing Non-Psychoactive Analogues: If the therapeutic benefit is solely derived from the dampening of the CeA’s stress response, future chemical research could focus on developing non-hallucinogenic compounds that maintain the highly targeted effect on CRF-R1 neurons without the subjective psychedelic experience.
- Combination Therapy: The study’s results lay the groundwork for potential combination therapies. For instance, combining psilocin with behavioral therapies or even other pharmacological agents that target different aspects of the addiction cycle (e.g., cravings or reward pathways) could yield synergistic effects.
Conclusion: A Calmer Future for Addiction Therapy
The preclinical study from the University of North Carolina at Chapel Hill has provided invaluable mechanistic insight into one of the most exciting new frontiers in addiction medicine. By demonstrating that the psychedelic metabolite psilocin can effectively dampen the pathological hyperactivity of stress-related neurons in the central amygdala of alcohol-exposed mice, the researchers have illuminated a direct pathway by which these compounds can reduce the compulsive drive to drink.
This discovery moves beyond mere correlation, establishing a foundational neurobiological explanation for the clinical promise of psychedelics in treating not only Alcohol Use Disorder but also co-occurring disorders like anxiety and depression. As this research transitions toward clinical application, the focus on restoring a calmer, more regulated emotional core in the brain offers a profound and optimistic vision for the future of addiction therapy. The goal is no longer just sobriety, but the restoration of emotional well-being—a calmer future awaits those struggling with the complex grip of AUD.