Introduction
Breast cancer remains the second leading cause of cancer-related death for American women, and the vast majority of these tragic outcomes—over 90%—are attributed not to the primary tumor, but to the metastatic spread of the disease. While advancements in detection and primary tumor treatment have significantly improved survival rates in recent decades, scientists continue to grapple with the molecular mechanisms that drive cancer cells to break away, travel through the body, and establish deadly secondary tumors, often while developing resistance to life-saving chemotherapies.
For years, research has hinted at an intriguing and concerning association between high levels of cholesterol and poor outcomes in breast cancer patients. This epidemiological link suggested that the body’s lipid metabolism might play a more direct, active role in cancer progression than previously understood. This suspicion was recently confirmed and defined by a groundbreaking discovery from the lab of Dr. Erik Nelson at the University of Illinois Urbana-Champaign Cancer Center (CCIL). Their work has successfully unmasked the precise molecular pathway that links cholesterol to cancer’s deadliest stages, revealing a malicious line of communication that hijacks the body’s own immune system to promote metastasis and drug resistance.
The Unmasking of the Culprit: 27-Hydroxycholesterol (27HC)
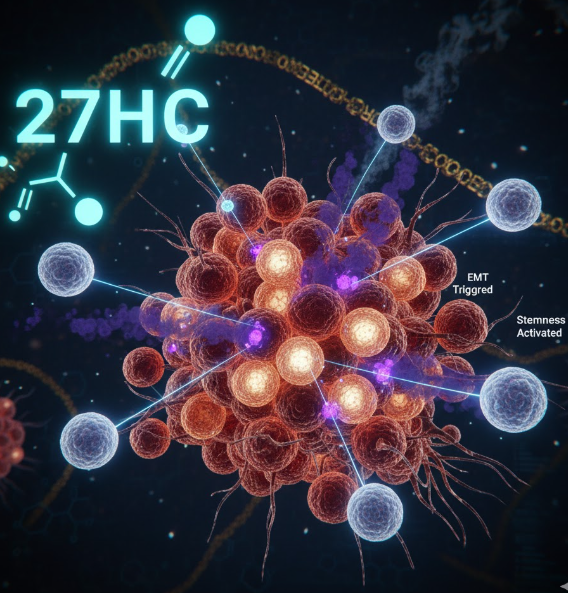
The general association between high cholesterol and increased cancer risk was a powerful clue, but the specific mechanism remained elusive. It wasn’t enough to know that cholesterol was involved; researchers needed to pinpoint which component was the active ingredient driving the cancerous changes. Dr. Nelson’s team, with first author Natalia Krawczynska, successfully narrowed the search, identifying a specific cholesterol metabolite as the true culprit: 27-hydroxycholesterol (27HC).
Cholesterol itself is vital for cellular health, serving as a building block for membranes and hormones. However, when cholesterol levels are elevated, the body produces various oxidized forms, or metabolites, in an attempt to process the excess. 27HC is one such metabolite. The researchers found that it is not simply passive collateral damage, but rather a powerful, highly active signaling molecule. Preclinical animal models confirmed that high levels of 27HC actively work to suppress the immune system’s ability to attack cancer cells, effectively lowering the body’s natural defense mechanisms. This discovery shifted the focus from merely reducing overall cholesterol to understanding and targeting the specific metabolic byproduct responsible for fueling cancer’s progression.
The Mechanism of Malicious Communication: Neutrophils and EVs
The team’s latest study, detailed in the journal Cancer Letters, describes the significant breakthrough: the definitive line of communication used by 27HC to instruct cancer cells to metastasize. This process is a complex cellular “telephone game” involving two key players: neutrophils and extracellular vesicles (EVs).
Neutrophils: From Defenders to Accomplices
Neutrophils are the most abundant type of white blood cell and serve as the first responders of the immune system, rushing to sites of infection or inflammation. In a healthy state, they are vital for combating pathogens. However, in the presence of high 27HC, their role is catastrophically altered.
The research demonstrated that the 27HC metabolite directly acts upon these neutrophils. Essentially, 27HC “tells” the neutrophils what to do—and that instruction is not to fight the cancer, but to prepare a package of pro-tumor instructions.
Extracellular Vesicles (EVs): The Cancer Courier System
The “package” is delivered via Extracellular Vesicles (EVs). EVs, sometimes referred to as exosomes or microvesicles, are tiny, lipid-membrane-bound spheres secreted by nearly all cell types. They act as the cellular postal service, carrying a cargo of proteins, lipids, and genetic material (like microRNAs) from one cell to another, facilitating communication within the tumor microenvironment.
In this pathological pathway, 27HC stimulates the neutrophils to secrete EVs with a highly specific, malignant cargo. The 27HC metabolite essentially customizes or “reprograms” the neutrophil-derived EVs, loading them with specific molecular instructions that promote tumor growth and spread. These customized neutrophil-EVs then travel through the bloodstream, reaching the cancer cells themselves.
Hijacking the Cancer Cell: Stemness and Epithelial-Mesenchymal Transition (EMT)
The moment the specialized neutrophil-EVs dock with the breast cancer cells, they deliver their harmful message, fundamentally altering the cancer cell’s identity and behavior. The central message of the EV cargo instructs the cancer cells to change their “makeup” and engage in pathways that facilitate aggression.
This transformation is known as the Epithelial-Mesenchymal Transition (EMT). Epithelial cells are typically stationary, structured, and adherent—characteristics of the initial, localized breast tumor. Mesenchymal cells, conversely, are migratory, invasive, and loosely organized. The transition is a crucial step for a stationary tumor cell to gain the characteristics necessary for metastasis. It allows them to detach from the primary tumor, enter the circulation (a process called intravasation), survive the journey, and set up colonies in distant organs (the extravasation and colonization process).
Furthermore, the EV messaging pushes the cancer cells toward a “stem-like” state, a condition often associated with the most aggressive and treatment-resistant cancers. Cancer stem cells are a small subpopulation within a tumor that possess the unique ability to self-renew and give rise to all the other cell types in the tumor, making them highly resistant to standard therapies. By driving cells toward this stem-like phenotype, the 27HC-neutrophil-EV axis creates a scenario where standard chemotherapies, which typically target rapidly dividing cells, become ineffective.
In essence, the cholesterol metabolite 27HC, through the medium of the neutrophil-EVs, acts as an accelerant, conferring two deadly traits upon the cancer: the ability to spread and the ability to survive treatment.
Therapeutic Horizon: Disrupting the Message
The discovery of this precise molecular axis provides a powerful new therapeutic opportunity. As Dr. Nelson noted, “We show that EVs from neutrophils instruct cancer cells to engage in pathways making them resistant to chemotherapies. That means, if we can disrupt this process, we can offer a solution to patients with current metastatic disease—making their current medications work better.”

The most promising implication of the research lies in the potential for reversibility. The scientists discovered that when this malicious communication pathway—the “message”—is eliminated, the cancer cells can revert to being more stationary and sensitive to medications. This suggests that a successful intervention strategy might not have to kill the cancer cells outright, but simply disarm them, making them vulnerable to existing treatments.
Targeting this axis presents several novel avenues:
- Inhibiting 27HC Synthesis: Drugs (like statins, already used to lower cholesterol) or new compounds could be developed to specifically block the enzyme responsible for creating 27HC from cholesterol.
- Blocking EV Secretion/Uptake: New chemical agents could be designed to prevent the 27HC from programming the neutrophils, prevent the neutrophils from releasing the EVs, or block the cancer cells from receiving the EV message.
- Neutralizing the EV Cargo: An alternative approach would be to identify and neutralize the specific toxic molecules (e.g., microRNAs or proteins) contained within the EVs that trigger the EMT process.
These strategies offer the hope of combining novel targeted therapies with conventional chemotherapies, transforming resistant, metastatic disease into a more manageable, stationary, and treatable condition.
Future Research and Clinical Applications
Building upon this foundational insight, the CCIL team is mapping out an intensive basic science research plan with multiple future directions:
- Drug Development and Screening: The lab will collaborate with chemists to screen existing compounds and develop entirely new molecules specifically designed to alter the neutrophil-EV communication loop. This work will require intense pre-clinical studies to evaluate efficacy and safety.
- Lifestyle and Diet Modulation: The researchers plan to investigate whether common dietary factors or existing drugs used for other conditions could inadvertently, or intentionally, modulate this neutrophil-EV communication axis. This opens the door to potential preventative or supplementary strategies that utilize lifestyle changes to lower metastatic risk.
- EVs as Prognostic Biomarkers: A critical clinical goal is to establish collaborations to determine whether monitoring the levels of these specific, customized EVs in the blood could serve as a non-invasive tool to predict metastatic recurrence in breast cancer patients. Identifying high-risk patients early based on circulating EVs would allow clinicians to intervene with preventative strategies or aggressive therapies before the metastatic disease clinically appears.
This comprehensive investigation underscores the interdisciplinary nature of modern cancer research, requiring the expertise of biologists, chemists, engineers, and computational biologists to translate a fundamental discovery into a clinically meaningful advancement.
Conclusion: A New Era of Targeted Cancer Prevention
The discovery by the University of Illinois Urbana-Champaign Cancer Center is a pivotal moment in oncology. By dissecting the cholesterol-to-cancer signaling pathway, researchers have provided a fundamental insight into how the tumor microenvironment is corrupted by a common lipid metabolite. They have exposed the mechanism by which 27HC transforms immune cells (neutrophils) into accomplices that dispatch malignant instructions (via EVs) to turn breast cancer cells into aggressive, chemoresistant stem-like invaders.
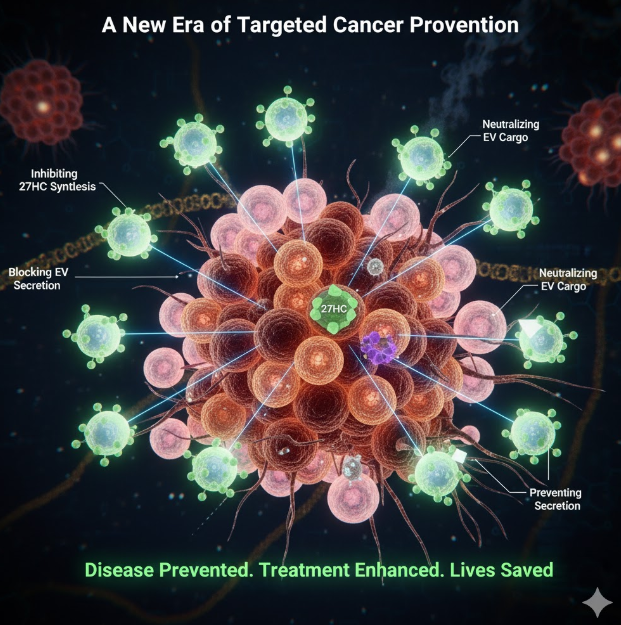
The clear and actionable target—disrupting the neutrophil-EV communication—offers a profound new direction for therapeutics. This research promises to move beyond simply treating metastatic disease to finding ways to prevent it from occurring in the first place, or, at the very least, rendering it sensitive to existing medications. This discovery offers genuine hope: by eliminating the malignant “message,” scientists can potentially neutralize one of the key drivers of breast cancer mortality, ushering in a new era of targeted prevention and more effective treatment.