Each year, influenza poses a significant global health threat, affecting millions of people worldwide. To combat this seasonal virus, the World Health Organization (WHO) recommends updated strains for the influenza vaccine, based on surveillance and the evolving nature of the virus. For the upcoming 2025-2026 Northern Hemisphere flu season, WHO has outlined changes in the vaccine composition to ensure greater protection against circulating strains. These updates reflect the continual adaptation of the influenza virus, which is characterized by antigenic shifts and drifts. Understanding these updates is crucial to maintaining the effectiveness of influenza prevention strategies.
The Role of WHO in Influenza Vaccine Strain Selection

The WHO plays a central role in selecting and recommending the strains that should be included in the seasonal influenza vaccine. The process of strain selection involves the collaboration of experts from the Global Influenza Surveillance and Response System (GISRS), which monitors influenza activity worldwide. This network provides real-time data on circulating strains, allowing WHO to recommend the most relevant strains for inclusion in the vaccine. The WHO’s expert committees review data from laboratories, health authorities, and vaccine manufacturers before making their recommendations.
Each year, the WHO evaluates the three or four influenza virus strains that are most likely to circulate in the upcoming flu season, selecting both the A (H1N1) and A (H3N2) subtypes of influenza A, as well as influenza B strains. Because the virus constantly evolves, it is essential that the vaccine is updated regularly to match the most prevalent circulating strains.
Key Updates for the 2025-2026 Influenza Vaccine Strain Recommendations
For the 2025-2026 Northern Hemisphere flu season, WHO has recommended several key updates to the influenza vaccine. The changes reflect the latest trends in global surveillance and are designed to provide more effective protection against the strains expected to dominate during the upcoming flu season.
- Influenza A (H1N1) Update: WHO has recommended a new lineage of the A (H1N1) virus for inclusion in the vaccine. This update is necessary because the A (H1N1) virus continues to evolve, and the previously recommended strain has shown reduced effectiveness against circulating variants. The new strain is expected to better match the virus circulating in the population and increase the vaccine’s efficacy.
- Influenza A (H3N2) Update: The A (H3N2) strain has historically been one of the more challenging strains to predict and vaccinate against due to its ability to mutate rapidly. For the 2025-2026 season, the WHO has recommended a new variant of the A (H3N2) virus. This update is based on genetic analyses showing that the current vaccine strain is no longer a close match to the viruses circulating in humans. The new strain should help improve immune responses and offer better protection.
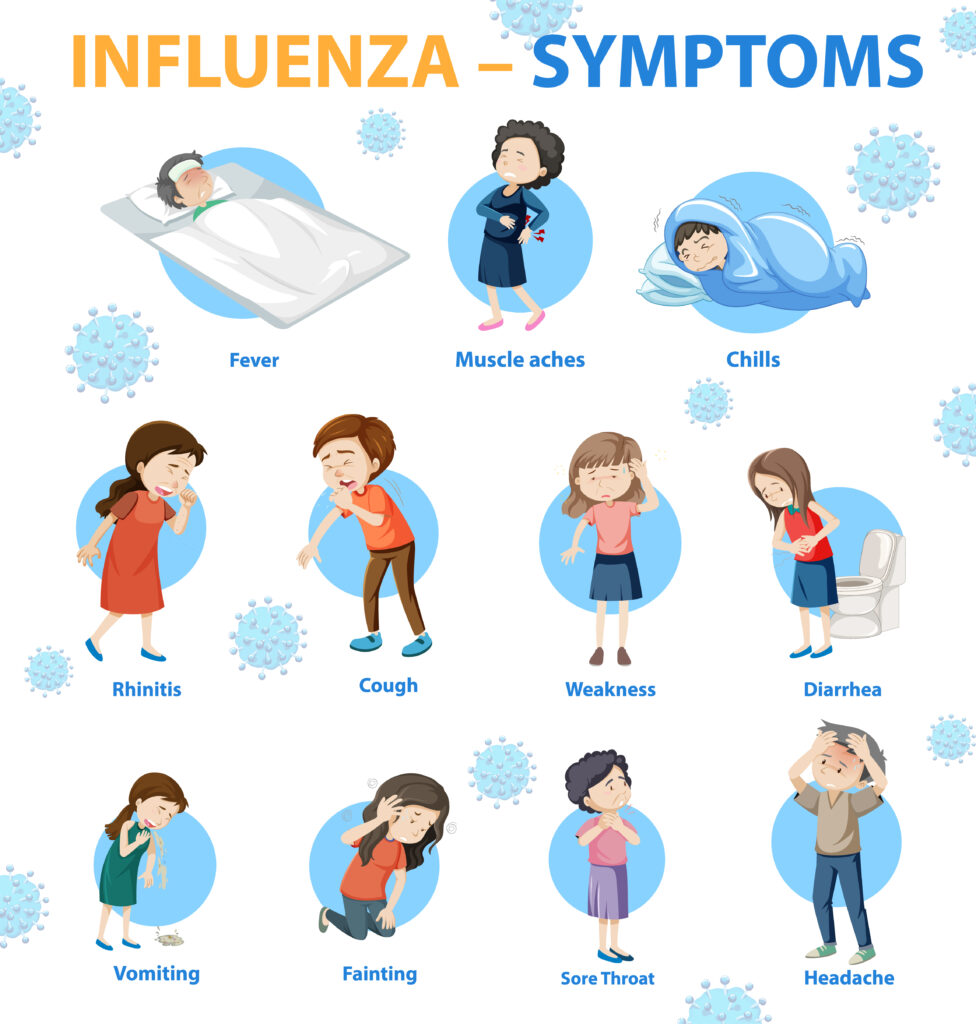
3. Influenza B Strain Selection: In addition to updates for influenza A strains, WHO has recommended changes to the influenza B component of the vaccine. The B/Victoria lineage has dominated in recent seasons, but new circulating variants have emerged. WHO has decided to replace the previous B strain with a more recent variant from the B/Yamagata lineage. This change aims to ensure that the vaccine can effectively combat the most relevant B strains circulating globally.
4. Potential Emergence of New Strains: One of the ongoing challenges in flu vaccine development is the unpredictable nature of the virus. Although the WHO’s recommendations are based on the best available data, there is always a possibility that a novel strain could emerge in the months leading up to the flu season. Surveillance systems continue to monitor this, and adjustments can be made if necessary
Why These Updates Matter
The influenza virus is known for its ability to change rapidly, which is why the vaccine needs to be updated annually. Antigenic drift, a process in which the virus undergoes small genetic changes, can render previously circulating strains less effective at evoking an immune response. By adjusting the vaccine composition to include the most current strains, WHO aims to increase the likelihood of immunity across the global population.
These updates are particularly important for high-risk groups, such as the elderly, young children, pregnant women, and individuals with underlying health conditions, who are more vulnerable to severe complications from the flu. Timely and accurate vaccine strain recommendations are critical in reducing the global burden of influenza and preventing widespread outbreaks that can strain healthcare systems.
The Global Impact of Influenza Vaccination
Influenza is a highly contagious respiratory disease that can cause severe illness and death. In the Northern Hemisphere, the flu season typically peaks between December and February, with the potential for outbreaks extending through the spring. The vaccine is the most effective tool we have in reducing the spread of the virus and minimizing the impact of flu-related hospitalizations and deaths.
In addition to protecting individuals, widespread vaccination efforts contribute to “herd immunity,” reducing the overall transmission of the virus within communities. For countries with robust vaccination programs, these efforts can prevent the spread of new strains and reduce the overall societal burden of the flu season.
The Future of Influenza Vaccines
Although current influenza vaccines are vital, their annual updating process presents certain challenges, particularly as the virus continues to evolve. Researchers are exploring the development of universal flu vaccines, which would provide long-lasting protection against a broader range of influenza strains. Such vaccines could simplify global vaccination efforts, especially in the context of rapid virus mutations.
For now, the 2025-2026 Northern Hemisphere vaccine update is a crucial step in continuing to protect populations worldwide from the flu virus. By following WHO’s recommendations and maintaining strong surveillance, we can improve flu vaccination strategies and save lives.
Conclusion
The WHO’s recommendations for the 2025-2026 Northern Hemisphere flu season are an essential part of global efforts to fight influenza. As the virus evolves, these updates ensure that the vaccine is as effective as possible in preventing illness. With continued surveillance and timely updates, we can reduce the global impact of influenza and safeguard the health of vulnerable populations.